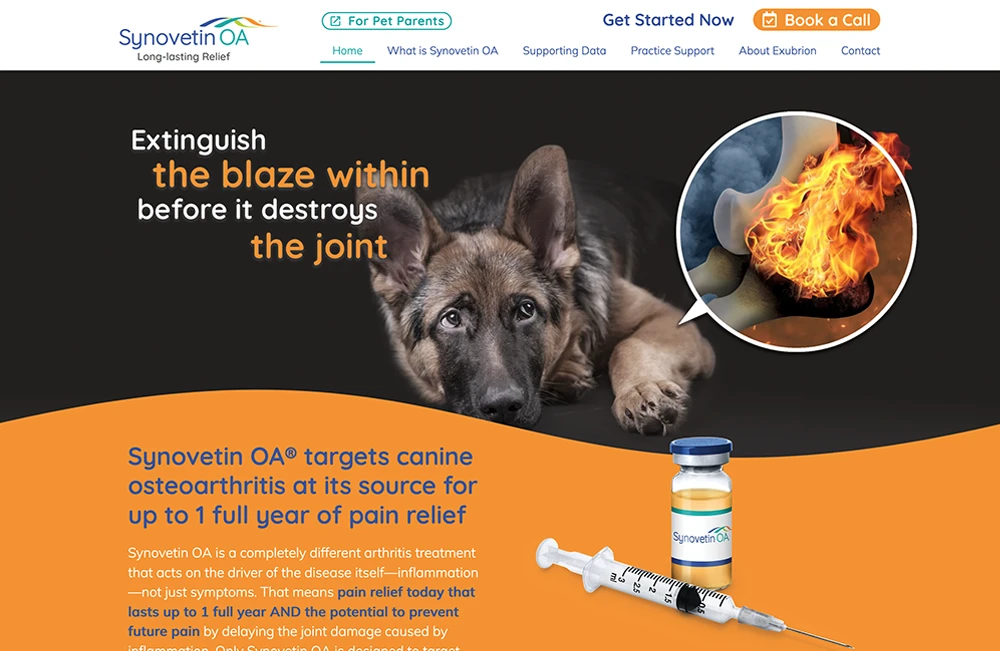
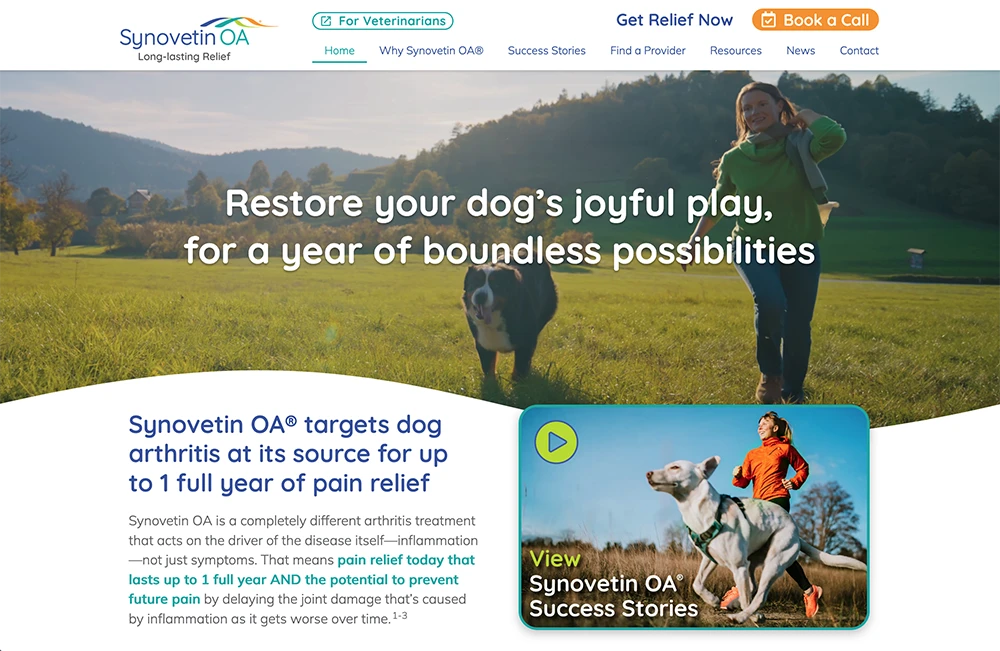
At Exubrion Therapeutics®, our vision is to forever improve the compassionate care of companion animals
Our name, Exubrion, emerged from the word “exuberance,” which is the quality of being full of energy and excitement.
- “Exubrion” speaks to our determination to help animals live healthy, active, and pain-free lives.
- “Exubrion” also identifies our enthusiasm for supporting those veterinarians who care for pets by providing new, innovative therapies, specifically for relieving the pain and suffering of canine osteoarthritis (OA).
Featured Press
APRIL 2025
Understanding Synovetin OA: A Breakthrough in Canine Osteoarthritis Treatment with Dr. Matthew Brunke
NOVEMBER 2024
Does your dog have arthritis? A lot of them do. But treatment can be tricky
APRIL 2023